About Coronavirus: The current 2019 coronavirus pandemic (COVID-19) is caused by a positive RNA virus, referred to as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Despite all the global efforts, the virus continues to spread and infect a large population and has affected 218 countries, globally, as of 11:08 am CEST, 20 May 2021, there have been 164,409,804 confirmed cases of COVID-19, including 3,409,220 deaths, reported to WHO. As of 18 May 2021, a total of 1,407,945,776 vaccine doses have been administered. India, the second most populated country, till 20 May 2021, there have been 2.58 crore confirmed cases of COVID-19 with 287,122 deaths, reported to WHO. As of 20 May 2021, a total of 18.6 crore vaccine doses have been administered. 4.11 crore have been fully vaccinated, and 3% of the population is fully vaccinated.
A necessity for a vaccine: VACCINES ARE ONE OF THE MOST SUCCESSFUL AND COST-EFFECTIVE HEALTH INVESTMENTS IN HISTORY. From Jenner and Pasteur to modern-day medicine, immunization has an impressive track record. The Coronavirus Pandemic is going on for a year and a half for now, and still, the end doesn’t seem to be nearer, at the most, it feels that this is just a beginning to the end as vaccine development and dispersal have begun. Scientists around the world are working faster than ever to develop and produce vaccines that can stop the spread of COVID-19. For COVID 19 vaccination hesitancy was common, as 46% would like to get the vaccine but would wait until others receive it. However, 14% don’t want to get the vaccine.
Since the emergence of this novel coronavirus in December 2019, more than a dozen vaccines have started to be rolled out. Here is an at-a-glance overview of those vaccines and recent developments of those vaccine candidates in clinical trials. There are more vaccine candidates simultaneously in the pipeline for COVID-19 than ever before for an infectious disease. All of them are trying to achieve the same thing – immunity against the virus, and some might also be able to stop transmission. They do so by stimulating an immune response to an antigen, a molecule found on the virus. In the case of COVID-19, the antigen is typically the characteristic spike protein found on the surface of the virus, which it normally uses to help it invade human cells.
Types of vaccine: THE FOUR MAIN TYPES OF COVID-19 VACCINE
There are four categories of vaccines in clinical trials:
- WHOLE VIRUS
- PROTEIN SUBUNIT
- VIRAL VECTOR and
- NUCLEIC ACID (RNA AND DNA).
Some of them try to smuggle the antigen into the body, others use the body’s cells to make the viral antigen. The main goal of vaccine development is Efficacy and Safety. “Efficacy of vaccine” is explained as the effectiveness of the vaccine to prevent infection by the virus or the ability to prevent the severity of disease (primarily) as the COVID spectrum ranges from asymptomatic to severe infection leading to ICU admission.
Phases of Vaccine development
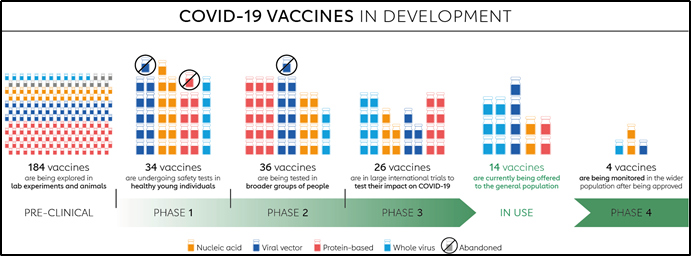
Pre- Clinical phase-Animal testing- The vaccine is given to an animal e.g. mice, then it is monitored for side effects like skin irritation, the efficacy of vaccine-like days after antibody development is seen after a dose, then the mice is infected by the virus, and then we check if the vaccine can prevent the disease by monitoring for symptoms and doing a PCR test, if the vaccine is successful then it will go to next phase.
Clinical Trial/ Human trial-
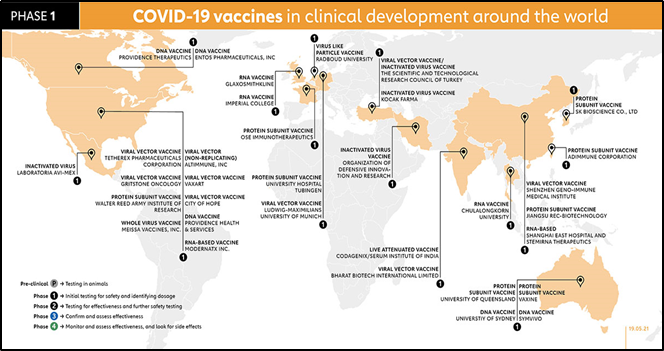
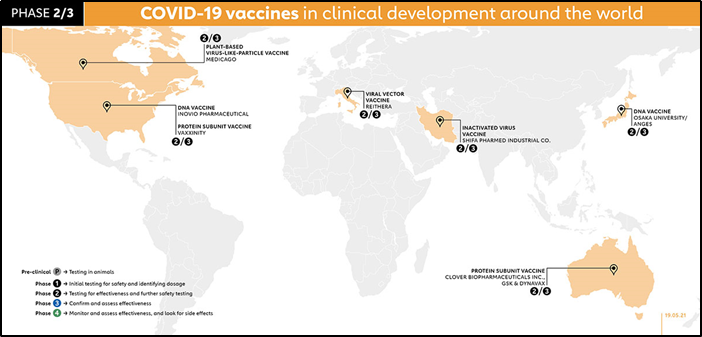
Phase 1- Small population/ sample size (<100 participants) This phase is primarily to test the vaccine’s safety, determine dosages and identify any potential side effects in a small number of people. It is given to healthy patients only who have no co-morbidities, then the subjects are tested for side effects, the effective vaccine dosage is noted as high dosage may have more side effects or lower dose- may not be effective or maybe develop not adequate antibody response. The vaccines in Phase 1 all around the world are illustrated in the picture below.
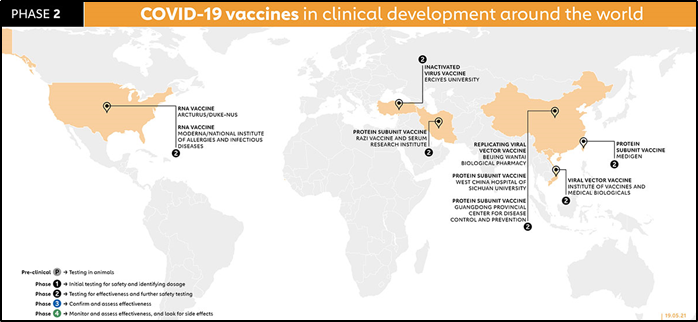
Phase 2 further explores the safety and starts to investigate efficacy on larger groups. It is taken on a larger population-moderate sample size (100-1000), in this phase, the patient's pool is tried to match the demographic of the population age-wise, race, gender, underlying comorbidities for better representation. The results are analyzed for side effects of vaccination, dosage of phase 1 is effective. Vaccines in Phase 2 are Arcturus/Duke NUS, Moderna, Razi, Inactivated Virus vaccine, Beijing Wantan, West China Hospital, Guangdong Provincial Center, Viral vector vaccine, Medigen.
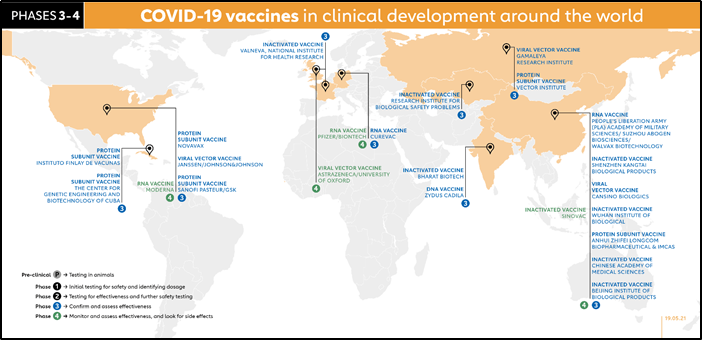
Phase 3 trials which few vaccines ever make it to, are much larger, involving thousands or tens of thousands of people, to confirm and assess the effectiveness of the vaccine and test whether any rare side effects only show up in large groups. The Sample pool is matched the demographic of the population, try to the monitor effectiveness of the vaccine in a real-life scenario, the investigators monitor for subjects for symptoms and by swab test for disease and infection. The control group is also tested who are not given a vaccine but a placebo. The efficacy of the vaccine is calculated and recommended for approval.
Phase 4 trials, is conducted after national regulatory approval and involves further monitoring in a wide population over a longer timeframe as a form of post-marketing surveillance (pharmacovigilance) by FDA or EMA (Emergency Medical approval)
Mechanism of how does the Vaccine function and provide protection
| Vaccine action mechanism | Name of vaccine |
| m RNA technology | Pfzier BioNTech, Moderna, Gennova/HDT Bio |
| Adeno Virus Vector | Oxford Aster Zeneca, Sputnik V, J&J |
| Killed Whole Viron | Covaxin( Bharat Biotech), Sinovac, SinoPharm |
| Protein subunit (Nanoparticle) | Novavax |
| DNA plasmid | Zydus (Cadilla) |
| Peptide Multiope | United Biomedical Taiwan |
| Live Attenuated Intra Nasal | Codagenix/SII |
| Virus-Like Particles (VLP) | Medicago, Spybiotech/SII |
- Whole virus vaccine: Many conventional vaccines use whole viruses to trigger an immune response. There are two main approaches. Live attenuated vaccines use a weakened form of the virus that can still replicate without causing illness. Inactivated vaccines use viruses whose genetic material has been destroyed so they cannot replicate, but can still trigger an immune response. Both types use well-established technology and pathways for regulatory approval, but live attenuated ones may risk causing disease in people with weak immune systems and often require careful cold storage, making their use more challenging in low-resource countries. Inactivated virus vaccines can be given to people with compromised immune systems but might also need cold storage.
a) Moderna and Pfizer/BioNtech- m RNA technology: It is a lipid nanoparticle i.e. phospholipid biolayer-acts as a vehicle for transporting m RNA of the SARS CoV2 virus. The S protein is most pathogenic and therefore the mRNA of S protein is used in this vaccine. This nanoparticle infects the host cells, releases the m RNA in these cells, however, it is not incorporated in the nucleus; instead, it uses cytoplasmic ribosomes and makes proteins i.e. (translation). These S proteins produced are then expressed on this host cell by MHC II protein which attracts B cells, macrophages, and dendritic cells and receptor-expressing these proteins are MHC I receptors which are found on all nucleated cells of the body. These attract the T helper cells (Th cells). These interact with TCR with help of CD4 protein leading to activation of these T cells and leading to cytokine release like IL 2 IL 4, IL 5. These cytokines cause the proliferation & differentiation of B cells to plasma cells. The plasma cells then produce the antibodies against the S protein of the virus which binds to this protein and neutralizes the virus causing its destruction. The cytokine also stimulates T helper cells and this causes a lot of memory B cells formation. The cytotoxic T cells interact with MHC I cells with CD 8 protein, this complex releases cytokines to destruct the infected cell (not the cells producing the response) and generate B cells and plasma cells. The duration for which this antibody lasts and effective is under study.
b) AstraZeneca /Oxford, Sputnik V- These vaccines use chimpanzee adenovirus to house a nucleic acid (DNA molecule). The DNA latches on the host cell, then the DNA moves inside the cell into the cytoplasm, then it migrates into the nucleus, but it doesn’t gets incorporate into host DNA but uses DNA enzymes to make mRNA, which attaches to cytoplasmic ribosomes and make proteins. These are expresses on host cells by MHC I and MHC II receptors. After this, the neutralizing mechanism is the same as Moderna
2. Virus-Like Particles (VLPs) This mechanism uses molecules that resemble the virus but without the virus genes. The molecules used are from animal, plant, bacteria, and yeast cell linage. The molecules after entering the cells self-assemble themselves and help to express synthesized viral proteins. This viral assembly is non replicating and highly immunogenic. This mechanism is used to administer HPV vaccines in females. This has the potential to be a polycorona vaccine.
3. Protein subunit: Subunit vaccines use pieces of the pathogen - often fragments of protein - to trigger an immune response. Doing so minimizes the risk of side effects, but it also means the immune response may be weaker. This is why they often require adjuvants, to help boost the immune response. An example of an existing subunit vaccine is the hepatitis B vaccine.
4. Viral vector vaccines: It also works by giving cells genetic instructions to produce antigens. But they differ from nucleic acid vaccines in that they use a harmless virus, different from the one the vaccine is targeting, to deliver these instructions into the cell. One type of virus that has often been used as a vector is adenovirus, which causes the common cold. As with nucleic acid vaccines, our cellular machinery is hijacked to produce the antigen from those instructions, to trigger an immune response. Viral vector vaccines can mimic natural viral infection and should therefore trigger a strong immune response. However, since there is a chance that many people may have already been exposed to the viruses being used as vectors, some may be immune to it, making the vaccine less effective.
| Vaccine name | Mechanism of protection | Dosage of vaccine administered | Methodology of study for the vaccine | Results of Efficacy for disease and severity of infection | Temperature and Storage conditions for Vaccines and their availability |
| Moderna by Cambridge, Massachusetts, and funded by the National Institute of Allergy and Infectious Diseases (NIAID) | RNA vaccine | 2 doses, at Day 0 and Day 28 after 1st dose. 14 days later investigator checked for symptoms of COVID | Trials were for 30000 people. Placebo & vaccine administered. 185 positives in the Placebo group, of which 30 were severe cases. The vaccine group showed 11 positive cases but no severe cases. | Efficacy—2 endpoints calculated Chances of preventing disease-(184-11)/185=94.5% Chances of preventing a severe infection -100%-0%=100% | -20 degrees Celsius, require ultra-cold freezers. By end of 2021 one billion vaccines doses can be produced |
| Pfizer/BioNTech BNT162 vaccine, Germany | RNA vaccine | 2 doses, at Day 0 and 21 days after 1st dose. 7 days later investigator checked for symptoms and then test PCR | The trial was for a 43000 sample size. Placebo & vaccine was given. Blinded 162 cases positive in Placebo group with 9 severe infections The vaccine group had 8 positive cases and 1 severe case. | Efficacy—2 endpoints calculated Chances of preventing disease -(162-8/162=95% Chances of preventing a severe infection is calculated by [100%-(1/8 =12.5%)]= 87.5% | -70 degrees Celsius, require ultra-cold freezers, 2021 1.3 billion units can be produced |
| AstraZeneca ChAdOx1 vaccine, developed by the University of Oxford | Viral vector vaccine | 2 doses, Day 0 and Day 28 after 1st dose. 14 days later investigator checks for PCR | The study was done in UK and Brazil. In Brazil—9000 participants In Placebo, the meningococcal vaccine was given in the full dose The Vaccine group received a full 1 +1 dose. In the UK there were 3000 participants In Placebo group =!st dose was 1/2 the quantity, while in A vaccine group full 1+1 dose was given. A total of 131 positive cases were seen in both studies 0 severe cases of COVID were reported in the full study | Efficacy—2 endpoints calculated Chances of preventing a disease Brazil study - 62% UK study- 90% Average- 70% | 7-8 degrees Celsius, can be stored in the refrigerator. By 2021, 3 billion units of vaccine can be produced, if less dose is effective can be 4.5 billion units |
| Bharat Biotech, India BBV-154 | Simian adenovirus vector | Intranasal vaccine, single-dose, Broder immune response noted IgG, mucosal IgA and T cell response | Prevents infection and transmission | ||
| Novax/SII (Covovax) | Protein subunit with lipid nanoparticle vaccine with saponin Matrix M as adjuvant | Two doses | Efficacy up to 96.4% for Wuhan strain(original strain), 86% against Kent variant, 55% efficacious against S Africain variant. 100% effective against preventing severe disease. | 2-8 degrees Celcius | |
| Zydus Cadilla, ZyCoV-D | Plasmid DNA vaccine targets membrane protein | 3 doses, trial in 12-17 age group is done | Efficacy data expected till July 2021, | ||
| J&J /Biological E | Human Adenovirus vector | Single-dose | 66-72% efficacy | Easier logistics | |
| Codagenix /SII (Covi-vax), India | Live attenuated vaccine, with the immune response against all proteins of SARS CoV2 | Intranasal ,single dose | Possible efficacy against variants |
Limitations of current vaccines:
- Lower efficacy- mRNA has 90% while others have 50-90%,
- The protection offered against severe disease and death but not against transmission and milder disease.
- Lower efficacies may lead to the evolution of further mutations and stronger variants
- mRNA vaccines are extremely temperature storage requirements
- Thrombotic complications are a matter of concern in J& J /Biological E
Booster dose requirements
Currently, trials are going on for the duration and dose of vaccine booster doses. Moderna, Pfizer, Oxford/ AstraZeneca are testing variant-specific boosters. The vaccine booster help to increase vaccine efficacy, the longevity of immune response. Most likely the booster would be annually like the influenza vaccine. mRNA platform is a rapidly developing method that can be utilized against future variants who escape routine vaccine immune responses.
Other modes /types of vaccines:
Mix variants vaccine: Oxford has currently led a Co-Cov trial in variant vaccination, Oxford-Pfizer or Pfizer -Oxford combination of vaccinations are also been tried. However currently using combination vaccines increases chances of fever, myalgia, and pain at the injection site, headache, and fatigue. However, combination vaccines have chances of increasing vaccine efficacy. Com-CoV 2 has planned to include Moderna and Novavax.
Oral vaccines: The oral vaccines will be very interesting as they will be very easy and fast to administer. Currently, it is in the preclinical stage. The oral vaccine usually employs VLP or vector-based mechanisms to elicit the immune response. Currently, Premas, Oramed, Vaxart, Symvivo are in the market which is under development. These vaccines can generate Ig A & IgG response locally and systematically. The vaccine also increases CD 8 T cells. The advantage of these vaccines is easy storage and administration.
Common side effects of the vaccine
- Anaphylaxis
- GB syndrome
- A small percentage of pain at the site
- Fatigue
- Headache
- Joint pain
- Muscle pain.
Other frequently asked question
Is the vaccine safe?
Currently approved COVID-19 vaccines are considered to be safe and effective. Most side effects occur within 6 weeks of someone getting the vaccine. FDA has required 8 weeks of safety monitoring. FDA advises a minimum of 3,000 participants to assess safety. Current studies have 30,000 to 50,000 participants. This demonstrates how safety is a top priority for the FDA and the healthcare community.
Should we trust health experts and the government when the vaccine was developed so quickly?
YES. The FDA is using the same standards as it has for years. No steps are being ‘skipped’ in vaccine development or safety evaluation. There are two advisory committees: The Vaccine and Related Biological Products Advisory Committee (VRBPAC) that advises the FDA. The Advisory Committee on Immunization Practices (ACIP) advises the CDC.
What is an emergency use authorization (EUA) and what does that mean for me?
An Emergency Use Authorization (EUA) for a vaccine is based on the need to use a vaccine quickly to save lives during a public health emergency. EUA is a shorter process but no steps are skipped in the safety evaluation process. The FDA will assess if the vaccine's known and potential benefits outweigh the known and potential risks. Both advisory boards (VRBPAC and ACIP) will also review all the data and make recommendations. A EUA does not mean that authorization was done too quickly or that the vaccine is not safe
How soon after the second vaccine will I be protected, and for how long?
Most of the vaccines are 2 doses, 3-4 weeks apart. Protection begins 1-2 weeks after the second dose. We will most likely not know how long the vaccine will be protective when we receive it. More research is needed. We may need to have vaccine shots for COVID-19 regularly (like the flu shot) but we do not know this yet
Will I still need to wear a mask?
Yes! Current vaccines are about 90-95% effective. Wearing a mask, practising social distancing and consistent hand washing will protect others, especially nursing home residents.
I’d rather wait until other people (my coworkers) have gotten the vaccine and see how they do; then I’ll consider getting it during the second clinic. Is that ok?
We appreciate this concern. The good news is that tens of thousands of people have already been vaccinated. Many eligible people must get vaccinated as soon as possible to achieve the best results. Having staff vaccinated on two different days allows people to cover for one another in case someone is out for a day due to side effects. The longer each person waits to be vaccinated, the greater the chance of contracting COVID-19 illness and possibly spreading it to nursing home residents, coworkers or family members.
Could someone in my family (household) ‘catch’ COVID-19 from me if I get vaccinated?
No. There is no evidence that people who have received the vaccine are contagious or can spread COVID-19 to family or household members due to the vaccine. However, it is possible that someone is infected with COVID-19, asymptomatic, and could spread the virus to others unrelated to receiving the vaccine. Standard recommendations apply within households to prevent spread: wear a mask; keep at least six feet apart from one another; use frequent, thorough handwashing; clean/disinfect frequently used surfaces such as light switches and door handles
I am pregnant – is the COVID-19 vaccine safe during pregnancy?
If someone pregnant is part of a group designated to receive the vaccine (e.g., healthcare workers), she may choose to be vaccinated. There is limited data on vaccine safety in pregnancy; however, pregnancy by itself is not a contraindication at this time. It is recommended that each person discuss the risks and benefits of vaccination with their healthcare provider
I am breastfeeding – is the COVID-19 vaccine safe during breastfeeding?
There is no data on vaccine safety during lactating or breastfeeding. It is recommended that each person discuss vaccination with their healthcare provider
Can the vaccine lead to infertility?
There is no data yet on fertility after the vaccine. The vaccine has not been associated with infertility. It is recommended that each person discuss options with their healthcare provider
I am under 18 years old – can I still get the vaccine?
The Pfizer-BioNTech vaccine has been approved for ages 16 and up. The Moderna vaccine has been approved for ages 18 and up. Studies are being done to determine whether or not the vaccine is safe in younger children. This guidance may be updated soon – please check back for updates. Most states require parental consent for someone under age 18 to receive the vaccine.
Are there medical or other contraindications to getting the vaccine?
There are a few serious health conditions that may suggest someone should not take the vaccine. Most people with chronic health conditions are still recommended to receive the vaccine. Certain allergies or a history of severe allergic reactions to ingredients in the vaccine may be a contraindication to receiving the COVID-19 vaccine. These questions should be discussed with your healthcare provider.
If I have already had COVID-19 and recovered, do I still need to get vaccinated with a COVID-19 vaccine?
Yes, you should be vaccinated regardless of whether you already had COVID-19. That’s because experts do not yet know how long you are protected from getting sick again after recovering from COVID-19. Even if you have already recovered from COVID-19, it is possible—although rare—that you could be infected with the virus that causes COVID-19 again. People who have had Covid in past must go for Covid Vaccination four to six weeks after recovery
If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, you should wait 30 to 90 days before getting a COVID-19 vaccine. Because during this period the preformed antibodies transfused in external plasma will wane off and this will avoid the neutralization of virus (protein) produced by the COVID vaccine. Talk to your doctor if you are unsure what treatments you received or if you have more questions about getting a COVID-19 vaccine. Experts are still learning more about how long vaccines protect against COVID-19 in real-world conditions. CDC will keep the public informed as new evidence becomes available
Which is a better vaccine to take?
All approved vaccines including Pfizer, Moderna, Covishield and Covaxin have ~100% efficacy in preventing Death due to Covid, And Very High efficacy against Severe Covid, High to moderate efficacy (60%-95%) against symptomatic Covid but poor efficacy only against asymptomatic covid so people should not run after efficacy data while choosing a vaccine
Why is it important for the maximum population to get vaccinated?
Because all vaccines prevent severe covid and death. Vaccination of a large cohort of population is important if we want to save humanity from I'll effects of the current pandemic. Everyone should get vaccinated and encourage others to get vaccinated. Data is emerging that they might need just one shot of vaccine as Robust Neutralising antibody titres and Strong T cell responses have been found in them even after a single shot of vaccination.
Are there any non -parenteral routes for vaccination?
Nasal vaccines might be able to prevent even asymptomatic covid because it generates local IgA antibodies cutting chain of transmission and bringing an end to this pandemic
Is tracking Immunity important after vaccination?
There are many people with a compromised immune system and some may not have adequate antibody response even after vaccination. People who are diabetic, elderly or even frail individuals in some cases, may not produce an adequate amount of antibodies and therefore it becomes important to track their level of immunity after the vaccination.
Giving an example, suppose there is a plasma donor to a COVID-19 patient, it will be important to do this test because unless the donor has neutralizing antibodies the plasma may not be of much use to the patient. One needs to then examine whether they need to take an additional dose of vaccination (more than what is normally administered) but more importantly, they need to be doubly careful in taking the precautions to avoid any exposure because of the risks of a lower immune response in the body
After getting a COVID-19 vaccine, will I test positive for COVID-19 on a viral test?
No. Neither the recently authorized and recommended vaccines nor the other COVID-19 vaccines currently in clinical trials can cause you to test positive on, which are used to see if you have a current infection.
If your body develops an immune response—the goal of vaccination—there is a possibility you may test positive on some antibody tests. Antibody tests indicate you had a previous infection and that you may have some level of protection against the virus. Experts are currently looking at how COVID-19 vaccination may affect antibody testing results.
Who can take or who has to avoid vaccination?
People with an allergy to food, drugs, latex, and venom previous non-covid vaccine can safely take the covid vaccine. Only People with severe anaphylaxis to previous covid or non-covid vaccine should avoid covid vaccine. People with nasal allergy, Bronchial asthma skin allergy can be safely vaccinated
I am Diabetic, Should I get myself Vaccinated?
People with Diabetes should go for vaccination after taking food/breakfast
Which Drugs are to be avoided if one is to take the COVID vaccine?
People who are on Corticosteroids should decrease the dose to less than 7.5 mg per day if possible, for six weeks when taking the vaccine because higher doses act as immunosuppressive and may decrease immunity development. Inhaled steroids may not be tapered when taking the covid vaccine because the systemic bioavailability of inhaled corticosteroids is low.